
By Mr. Ukrit Sahapatsombut, Dr. Thanya Phraewphipat, Dr. Jiravan Mongkoltanatas, Dr. Priew Eiamlamai,
Dr. Nattanai Kunanusont, and Dr. Pimpa Limthongkul
Energy Storage Technology Research Team,
Energy Innovation Research Group of the National Energy Technology Center (ENTEC)
Overview
A:
Batteries in use today are divided into two main types:
1) Single-use batteries or primary batteries.
2) Rechargeable batteries or secondary batteries.
All batteries operate through electrochemical reactions called oxidation and reduction. Oxidation occurs at the anode. Reduction occurs at the cathode. In a single-use battery, these reactions are irreversible; whereas in a rechargeable battery, these reactions are reversible.
Q: What types of rechargeable batteries are in use today? And what are the differences?
A:
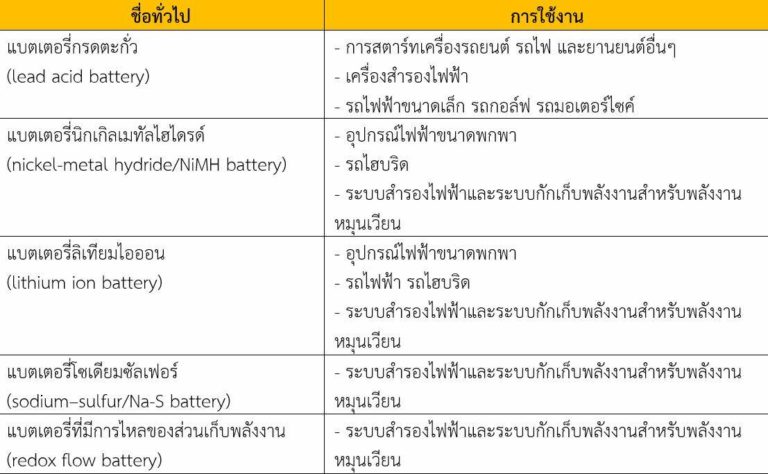
There are a dozen of rechargeable batteries but only a few are widely used, such as lead-acid batteries, nickel-metal hybrid batteries, lithium-Ion batteries, sodium-sulfur batteries, and batteries with the flow of energy storage as shown in Figure1.
Table1: Batteries in use today
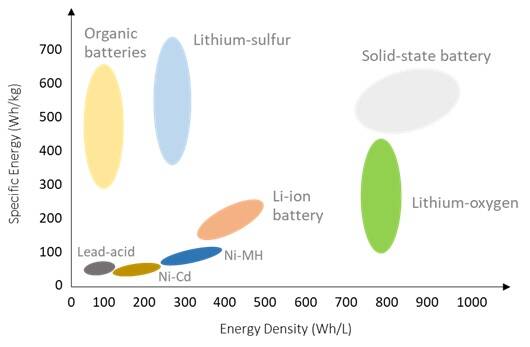
Q: What is the direction of the development for each type of battery?
A:
In general, the aim of battery development is to achieve higher energy density (either per weight or per volume), improved safety performance, and lower price. According to Figure 1, it can be seen that the direction of battery technology is to develop higher energy density and specific energy; some examples shown are lithium-sulfur battery, lithium-air battery, zinc battery, aluminum battery, and solid lithium-ion battery. [1]
In addition, the development also includes materials improvement, such as anode material, cathode material, and electrolyte solution (especially in lithium-ion batteries that have higher electrical energy and power), as well as an increase in the range of operating temperatures.
Q: Is it OK to use a mobile phone while charging the battery? Why?
A:
Using mobile phones while charging is possible but not recommended. [2] The reason is that using and charging a mobile phone at the same time from a household electrical power (220V) power may result in danger from an electric shock or a leakage. Not only this danger comes from mobile phones or batteries, but also they can be caused by other electrical appliances in the house depending on the quality of the equipment and preventive measures.
The reasons that a user can get an electrical shock from a mobile phone while charging are as follows:
1. The charger is defected or has low quality. The function of a charger (adapter) is to convert an alternating current (AC) of 220V into a direct current (DC) with a voltage of no greater than 5V and protect the user from getting the AC power directly. However, if the charger is defected, it may create an electrical leakage.
2. The charging cable has low quality. The charging cable that has been in use for quite a while may be torn or broken, increasing a chance for leakage and burning risk.
Therefore, using a mobile phone while charging with a household electrical power (220V) power may not be safe. However, if it is necessary, using a power bank is a better choice.
In addition, using a mobile phone while charging may affect the battery lifespan due to extra heat generated. Thus, the user should avoid using the battery while charging to preserve the battery
Q: How does the charging affect the battery lifespan? What is the correct way to charge the battery?
A:
There are various types of batteries, each has its own characteristics and should be treated appropriately.
A lead-acid battery should always be kept in a fully charged condition.
A nickel-cadmium battery and a nickel-metal hydride battery, should not be frequently charged but should be used until fully discharged and then recharged to its full capacity.
A lithium-ion battery should be charged with a constant current. In some devices, when the battery is nearly fulled (>80-90%), the regulator will reduce the electric current by charging with constant voltage until the battery is fully charged. However, in theory, the optimum operating range to extend the lifespan of lithium-ion batteries is 20-80%. Therefore, if the users want to extend its lifespan, the following issues are noted.
- A lithium-ion battery should not be used until it runs out because it will deteriorate faster.
- Charging a lithium-ion battery overnight normally does not affect its lifespan significantly; however, this also depends on the device and charging system used
- A fully-charged lithium-ion battery that has been left unused for a long time will deteriorate itself faster. Therefore, if not using, it should be charged halfway. However, if using the battery regularly, it can be fully charged in an appropriate way.
- A lithium-ion battery should not be charged and used simultaneously.
Most importantly, the factors that affect battery lifespan, in general, are temperature, electric current, and charging voltage. Some recommendations for preserving the battery charging are as follows.
- Choosing the right charger for the battery, e.g., a charger that has been approved to use with a particular device.
- Do not use encapsulated gadgets (such as cell phone cases) that trap heat while charging and the device should not be placed in an enclosed or unventilated area.
- Do not charge the battery in a high temperature location.
Q: What are the signs of battery deterioration?
A:
- The hours of battery life decreases.
Battery deterioration means the battery’s capacity decreases and less power is available for a fully-charged battery. That is to say, a fully charged battery has a shorter lifetime for a similar task. - The heat of the battery increases.
Once the battery deteriorates, the internal resistance of the battery will increase. That is, more heat will be generated compared to that of the new battery, and the user will feel the heat (e.g., while using a mobile phone). - The battery behaves differently.
When the chemicals or materials inside the battery deteriorate, it may cause an accumulation of gases inside the battery, resulting in a swollen battery with a possible leakage of electrolyte.
Q: What makes the battery degrade? How to prevent it? Are the viscous liquids dangerous, especially when they touch the skin or wound?
A:
Battery deterioration is caused by chemical deterioration within the battery cells that decreases the battery’s capacity. Therefore, less power could be used for a fully charged battery. Some possible causes of battery deterioration are as follows:
- An expiration of the chemicals or materials inside a battery cell.
- An improper use and storage, which generate heat, a most influential factor that accelerate the deterioration of the battery.
- A malfunction caused by undercharging or overcharging.
Thus, a practical preventive measure is to regularly inspect and check the charging system and examine the outside conditions and the leakage of the battery cell. In addition, a battery should not be stored or used in high temperatures since the battery lifespan will be reduced.[3]
The viscous liquids commonly seen leaking out from the carbon-zinc cell and alkaline cell are electrolytes. Typical batteries use ammonium chloride (NH4Cl) and zinc chloride (ZnCl2) as electrolytes. When manganese dioxide is completely reacted (the battery runs out), the acidic electrolyte will be able to react chemically with the zinc canister, which causes corrosion and leakage of internal chemicals. As for alkaline batteries, a potassium hydroxide solution is used as a basic electrolyte solution that can also react chemically with zinc containers when the battery has reached the end of its lifespan. Apart from the solution leakage, heavy metals were also found as shown in figure 2. An electrolyte of lithium-ion batteries is in liquid state and consists of lithium salts carbonate organic solvents. It can be deteriorated by moisture or heat, thus resulting in leakage occurs.
When an electrolyte is in contact with water or external moisture, it forms hydrofluoric acid (HF) with severely corrosive and highly toxic properties and can cause irritation. This acid can damage tissues and interfere with the function of the nervous system so that people who are in contact with this acid may not feel any pain at first, but in the long run, it will lead to osteoporosis and osteoarthritis. Thus, this acid is extremely detrimental.

Q: Does putting a battery in a freezer help extend the battery lifespan somehow?
A:
Putting a battery in a freezer in the hope of extending its lifespan is a misunderstanding because the freezer has a very low temperature, which may cause various terminals to deteriorate. That is to say, the battery should be kept at a temperature that is neither too high nor too low. However, once the battery is swollen or deteriorated, it should not be used but be replaced with a new battery.Q: What is the memory effect in the battery? In what types of batteries this effect be found? How does it affect battery performance?
A:
The reason why certain batteries have a memory effect or unwanted “memory” is that there is a change in the microstructures of the positive and negative terminals that have not been used for a long time. This effect is found in nickel batteries, such as nickel-cadmium or nickel-metal hydride. In general, the crystal size of nickel compounds that collects the electric charge is small, thus creating a large surface area to conduct the electricity. However, if a part of the battery is left unused, the crystal size will increase, and the terminals’ overall surface will decrease, as well as the electrical resistance will increase and the reaction becomes more difficult, leading to the deterioration of the battery performance.
To avoid a memory effect, nickel batteries should not be charged and used at the same time but it should be used until it runs out or drains at least once a month to keep the crystal size of the nickel compound in the anode close to that of the original. The most widely used solution relies on a device that can draw an electric current until the battery has an electric potential lower than usual or 0.4-0.6 volts per cell to allow the large crystal forming at the pole to split and become smaller.
Currently, Sanyo/Panasonic (Sanyo SMI-Thailand Co., Ltd.) has succeeded in reducing the memory effect in Ni-MH batteries under the brand ‘Eneloop’.
Q: What are the reasons that may cause a battery to explode or catch fire?
A:
Various incidents may result in battery explosion and are grouped as follows.
- A battery explodes or catches fire from external factors.
1.1 It is overheated (thermal abuse).
1.2 It is impacted by external shocks (mechanical abuse), such as being dropped or crushed, or impacted from a collision.
- A battery explodes or catches fire from internal factors.
- A battery management system has malfunctioned (BMS malfunction).
- A battery is overcharged (charger malfunction).
- An internal short circuit due to false design or the degradation of a battery.
- A battery explodes or catches fire from external factors.
These factors create internal heat and chemical reactions, leading to swollen, or exploded batteries if they cannot be cooled or released the gas. In order to prevent heat and high-pressure situations, most batteries should have expansion valves as a primary protection device. In addition, for lithium batteries with high-energy capacity, it is necessary to have protection devices preventing over-charged and over-discharged of the limited current and voltage.
There are two types of protection devices:
- Protection Circuit Module (PCM) to prevent an overcharge and overuse of the battery by terminating the electricity when the battery reaches the specified voltage level.
- Battery Management System (BMS) is a system, which controls and prevents the battery from working in danger, such as overcharging or discharging batteries, as well as balancing cell batteries to maximize battery capacity and extend its lifespan.
Q: What type of battery is a power bank? Why is it forbidden to carry on a plane if the capacity is larger than 32,000 mAh?
A:
A power bank contains lithium-ion batteries, which is forbidden to carry on a plane because it has a high energy-to-weight capacity. The International Air Transport Association (IATA) [4], therefore, has specified the rules and regulations of power bank batteries as follows.
- Do not put power bank batteries in checked luggage.
- The power bank batteries are allowed to carry a carry-on bag with a capacity limit of not more than 32,000 mAh.
- The power bank batteries with a capacity of 20,000 mAh (or less than 100 watt-hours (Wh) can be carried onboard up to 20 batteries.
- The power bank batteries with a capacity of 20,000–32,000 mAh (or between 100-160 watt-hours (Wh) can be carried onboard up to 2 batteries.
- The power bank batteries with a capacity of 32,000 mAh (or more than 160 watt-hours (Wh) are not allowed on board.
The 32,000 mAh number is specified based on the electrical power of a power bank with a 160 Wh at approximately 5 Volts (32,000 mAh x 5 V = 160 Wh), of which 160 Wh is equivalent to 1/7 of the energy of 1 kg TNT bomb. [5]
Battery management
Q: Are different types of batteries handled differently? How?
A:
Used batteries are hazardous waste that cannot be disposed of by landfill or incineration like conventional waste. This is cause batteries contain metal elements, which cannot be decomposed naturally by landfills and creates air pollution when incinerated. Batteries also contain acidic electrolytes or toxic organic substances, thus they may leak and create environmental pollution in a landfill. Therefore, specific measures have been taken to ensure a proper handling of the battery. Battery management starts with collecting used and worn-out batteries, for example, small batteries, such as batteries in mobile phones and computers. It is consumer’s responsibility to dispose of them at the correct e-waste collection point. These batteries should not be disposed with general waste because they may be incinerated, thus creating environmental pollution. As for large batteries, such as lead-acid batteries used in automobiles or lithium-ion batteries used in electric vehicles, the entrepreneurs in the industrial sector, such as battery dealers or electric vehicle dealers, are responsible for collecting and handling used batteries under the supervision of the Department of Industrial Works. A battery collection process is followed by a separation process, which will sort out the types of batteries for further disposal or recycling. The principles of handling or recycling each type of battery are similar, i.e., discharging to reduce the energy of a battery and make sure that it will not spark, and then dismantling all parts to separate the electrolyte and materials in the battery before disposing or recycling process. In other words, the difference in handling each type of battery is the disposing or recycling procedure of battery materials since different batteries have different components.Q: How should users dispose of the batteries to reduce their environmental impact? Is there a management system in Thailand? There are various Battery disposal points, and where do discarded batteries go?
A:
The user (or the consumer) should be aware of the proper disposal of the battery by separating the battery from the device in use first. Then put it in a sealed bag to avoid a short circuit and clearly label it as toxic waste, and discarded at various receiving points, such as department stores, mobile network shops, and post offices. However, if the battery cannot be removed, such as mobile phones batteries, large batteries (e.g., lead-acid batteries), or battery packs in electric vehicles, the consumers should return or replace the battery to the supplier and should not disassemble it to get valuable materials from the battery.
At present, lead-acid batteries are the most commonly used batteries in automobiles, therefore, the consumers or suppliers normally separate batteries to get the lead ingots. This will cause the smelters to receive lead poison through contact and respiratory tract, as well as creates environmental pollution. In addition, disposing of the acid that contains electrolytes inside the lead-acid batteries also directly impacts the environment.
In Thailand, no law specifically supports battery waste management, but there are laws related to electronic waste management, such as the Act (Act on Enhancement and Conservation of National Environment Quality B.E. 2535) that controls waste disposal and maintains environmental quality, the Factory Act B.E. 2535 that sets standards and methods for controlling waste from the factory, the Hazardous Substance Act B.E. 2535 that applies to those who manufacture, import, export or possess hazardous substances, and the Public Health Act (No. 2) B.E. 2550 that specify local administrative organizations must arrange for the proper separation, collection, and disposal of hazardous waste from the community. Currently, Thailand is in the process of pushing for a draft of the Act on the Management of Waste Electrical and Electronic Equipment B.E. (WEEE: Waste Electric and Electronic Equipment Act) [6]. This Act includes the Extended Producer Responsibility: EPR) and product recall systems, which allow an entrepreneur to retrieve more scrap batteries for recycling.
Q: Can batteries be recycled?
A:
Batteries can be recycled. There are various methods of recycling depending on the type and material inside the battery. At present, lead-acid batteries are in large quantities and mostly used in automobiles. The recycling of lead-acid batteries starts by pouring the acid out of the battery, then disassembling to separate the case and material for plastic cases to be recycled, and the internal material can be separated into lead oxides and plates, which are melted in the factory to smelt lead and recycle the elemental plates.
As for lithium-ion batteries, there will be more battery waste in the future due to the increased use of electric vehicles (xEV). It is estimated that by 2032, Thailand will have more than 22 million electric vehicles [7] , making the recycling of lithium-ion batteries a crucial topic in the future.
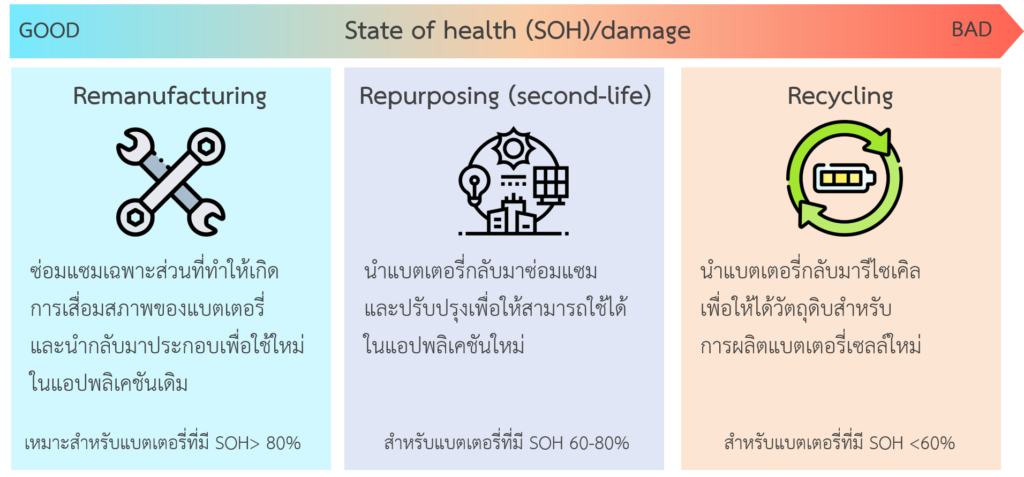
Since the material value of lithium-ion batteries accounts for 64% of the total battery value. [8] The deteriorated or failing lithium-ion batteries are checked for electrical performance and state-of-health of the battery first. If the battery is damaged in the electrical circuit or some cells in the module are damaged, it will be repaired or replaced, and then reused, which is called remanufacturing method. Moreover, if the battery has a State-of-health between 60-80%, it can be used in other applications that require less battery capacity, which is called battery repurposing or a second-life method, such as using batteries in deteriorated electric vehicles in stationary energy storage systems. Currently, Nissan Motor (Thailand) Company Limited has cooperated with Eaton Industries (Thailand) Ltd., to develop used lithium-ion batteries in Nissan leaf cars as a home backup power source, which is called xStorage. [9] However, if it was found that a state-of-health of batteries has a lower value than 60%, it can be recycled as shown in figure 3.
Battery recycling consists of four main processes:
- The mechanical process: a process of decomposing and separating battery materials by a mechanical process, such as crushing, filtering, magnetization, and refrigeration.
- The pyrometallurgical process: a process that uses heat to melt and smelt iron and bring back precious metals in the form of alloys.
- The hydrometallurgical process: a process that uses chemicals by leaching and returning precious metals in the form of a salt solution.
- The direct recycling process: a treatment for the material inside the battery that is directly deteriorated to make the deteriorated material to have a quality as original materials and can be used in the reassembled battery.
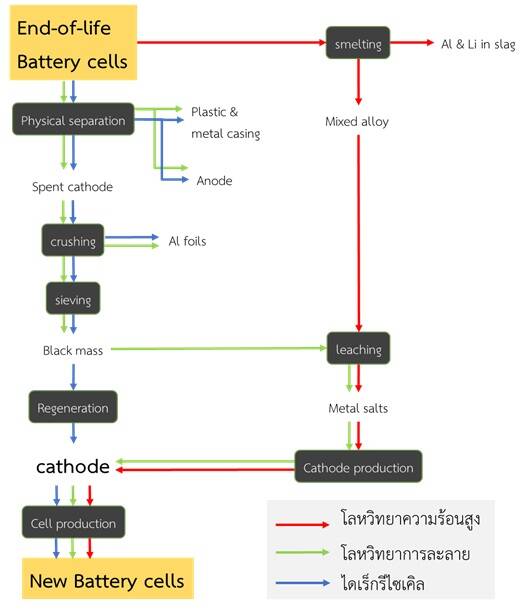
Recycling lithium-ion batteries involves a combination of the above processes [10] as shown in Figure 4, which each company from various countries has different ways to recycle lithium-ion batteries. [11] For example, the Umicore Company in Belgium, which has used the thermal metallurgy process in the smelting of precious metals such as cobalt, nickel, copper and iron into alloys and then melted by hydrometallurgical process to separate precious metals. As the Retriev Company in the United States, which has used liquid nitrogen to freeze batteries to prevent lithium reaction and then crush and separate the materials, it was then separated by hydrometallurgical process to to extract lithium and cobalt. In Thailand, there is no lithium-ion battery recycling plant, therefore, the entreprenuers will export used lithium-ion batteries to foreign countries for recycling.
References
- BATTERY 2030+ Roadmap: Inventing the Sustainable Batteries of the Future, Research Needs and Future Actions, https://battery2030.eu/digitalAssets/861/c_861008-l_1-k_roadmap-27-march.pdf (accessed November 5, 2020).
- เตือนภัยอย่าใช้มือถือขณะชาร์จแบต, การไฟฟ้าส่วนภูมิภาค, https://www.pea.co.th/ (accessed December 23, 2020)
- T.D. Tran, J.H. Feikert, R.W. Pekala, K. Kinoshita, Rate effect on lithium-ion graphite electrode performance, J. Appl. Electrochem. 26 (1996) 1161–1167. https://doi.org/10.1007/BF00243741.
- Traveling with Portable Electronic Devices (PEDs), https://www.iata.org/en/programs/ops-infra/baggage/ped/ (accessed November 27, 2020)
- Energy and Work Conversion Table, https://www.unitconversion.org/unit_converter/energy-ex.html (accessed December 29, 2020)
- บันทึกหลักการและเหตุผล ประกอบร่างพระราขบัญญัติการจัดการซากผลิตภัณฑ์เครื่องใช้ไฟฟ้าและอุปกรณ์อิเล็กทรอนิกส์ พ.ศ. …., https://www.lawamendment.go.th/index.php/faq?id=717 (accessed November 11, 2020)
- รายงานแผนพัฒนาโครงสร้างพื้นฐานด้านไฟฟ้าเพื่อรองรับยานยนต์ไฟฟ้าของประเทศไทย, https://www.eppo.go.th/images/Infromation_service/studyreport/EV_plan.pdf (accessed November 11, 2020)
- Pillot, Christophe. “Avicenne Energy.”Battery Market Compilations, twenty first ed. (2017).
- Nissan and Eaton release ‘Xstorage’ – home energy storage solution, https://www.pntpower.com/nissan-eaton-release-xstorage-home-energy-storage-solution/ (accessed November 11, 2020)
- M. Chen, X. Ma, B. Chen, R. Arsenault, P. Karlson, N. Simon, Y. Wang, Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries, Joule. 3 (2019) 2622–2646. https://doi.org/10.1016/j.joule.2019.09.014.
- Pinegar, Haruka, and York R. Smith. “Recycling of end-of-life lithium ion batteries, Part I: Commercial processes.”Journal of Sustainable Metallurgy (2019): 1-15.



